Synthesis of Mesoporous Alumina from Boehmite in the Presence of Triblock Copolymer
welcome inquiry email carrier@catalystcarrier
Abstract
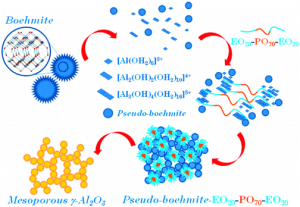
Mesoporous alumina was synthesized using commercial boehmite in the presence of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer Pluronic P123. Its calcination at 400°C yielded γ-alumina, in contrast to the ordered mesoporous alumina (OMA) obtained by hydrolysis of aluminum alkoxide in the presence of the same triblock copolymer. This synthesis afforded boehmite-based mesoporous alumina (BMA) with better adsorption properties and higher thermal stability in comparison to the alkoxide-based OMA, which remained amorphous after calcination below 900°C. The BMA materials also exhibited higher amount of acidic and basic sites as evidenced by ammonia (NH3) and carbon dioxide (CO2) temperature programmed desorption (TPD), respectively. Dispersion of commercial boehmite precursor under microwave irradiation afforded BMA materials with similar surface characteristics as those of the corresponding BMA samples obtained under conventional conditions, but showing slightly lower acidity and better basic properties. Thus, the dispersion method of boehmite can be used to modify the surface properties of the resulting BMA samples without sacrificing their porosity.
Keywords:
acidity; boehmite; mesoporous alumina; nitrogen adsorption; temperature programmed desorption; γ -alumina
